Management Recommendations for Dupilumab Partial and Non-durable Responders in Atopic Dermatitis | SpringerLink
Dupixent® 300 mg Injektionslösung in einer Fertigspritze Dupixent® 300 mg Injektionslösung im Fertigpen
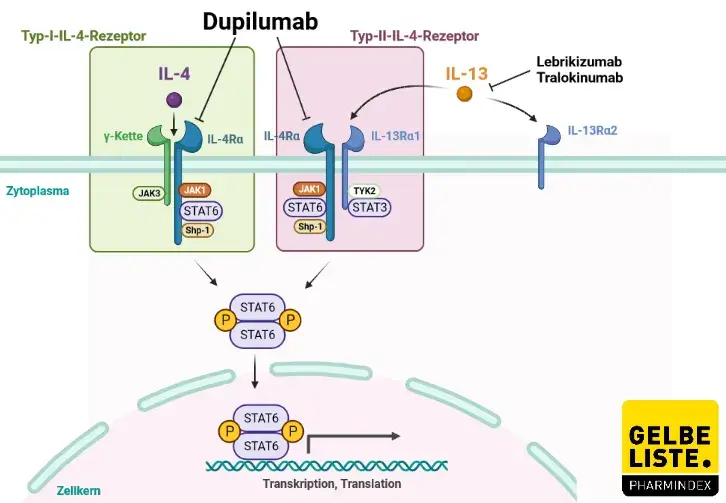
Effect of Dupilumab on Laboratory Parameters in Adolescents with Atopic Dermatitis: Results from a Randomized, Placebo-Controlled, Phase 3 Clinical Trial | SpringerLink

JPM | Free Full-Text | Proposal for a Structured Outpatient Clinic for Dupilumab Treatment in Chronic Rhinosinusitis with Nasal Polyps in the First Year of Treatment

Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial - The Lancet

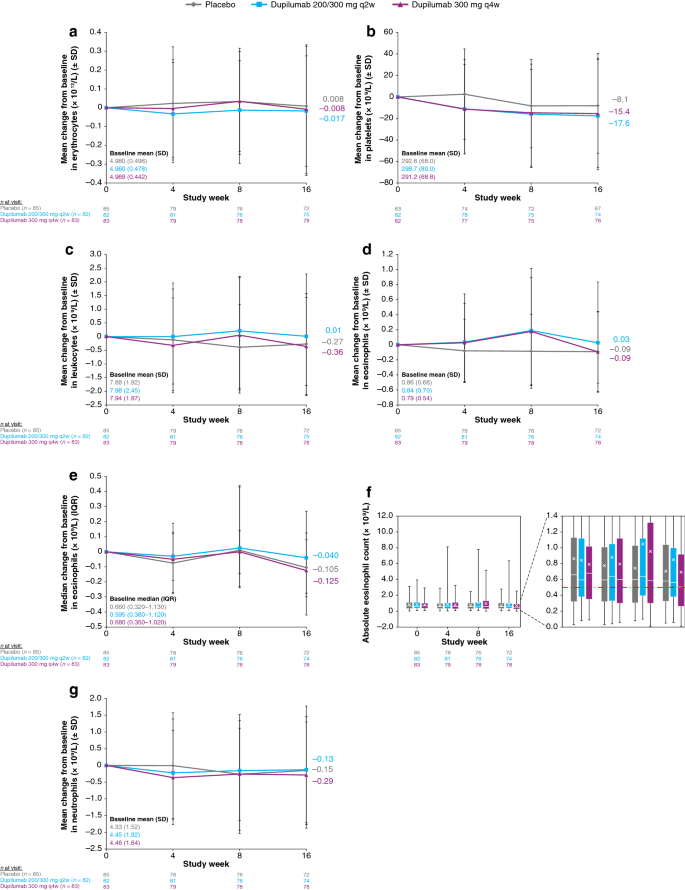
Laboratory Safety from a Randomized 16-Week Phase III Study of Dupilumab in Children Aged 6 Months to 5 Years with Moderate-to-Severe Atopic Dermatitis | springermedizin.de

Real-World Experience and Laboratory Monitoring of Dupilumab in Patients with Moderate to Severe Atopic Dermatitis in a Tertiary Centre | SpringerLink

JPM | Free Full-Text | Proposal for a Structured Outpatient Clinic for Dupilumab Treatment in Chronic Rhinosinusitis with Nasal Polyps in the First Year of Treatment

Handlungsempfehlung zur Therapieumstellung von Immunsuppressiva auf Dupilumab bei Patienten mit atopischer Dermatitis | SpringerLink